Never in the history of medicine have we been asked to define the natural history of a disease so quickly.
That’s according to David Pearce, Ph.D., who joined Sanford Health News for a Facebook Live event. Dr. Pearce is President of Sanford Research, Innovation and World Clinic.
Watch now: Facebook Live Q&A with Dr. David Pearce, Aug. 25
“With our research here and with our partners across the world, we have a much better understanding of what COVID is about now,” Dr. Pearce said. “We’ve been leading clinical trials so if you’re hospitalized, we have options for you. If you’re intubated, we have options for you.”
Research is critical during this pandemic, and Dr. Pearce said Sanford Research, with its cutting-edge technology, is well-positioned to advance the studies of antibodies, treatments and more.
COVID-19 clinical trials
While many consumers focus on the timeline for a vaccine, Sanford Health researchers are focused on treatment for patients who have tested positive for COVID-19. Dr. Pearce says clinical trials are an additional treatment option and explains the four phases.
Listen now: Dr. Pearce breaks down the four phases of a clinical trial
Enlarge
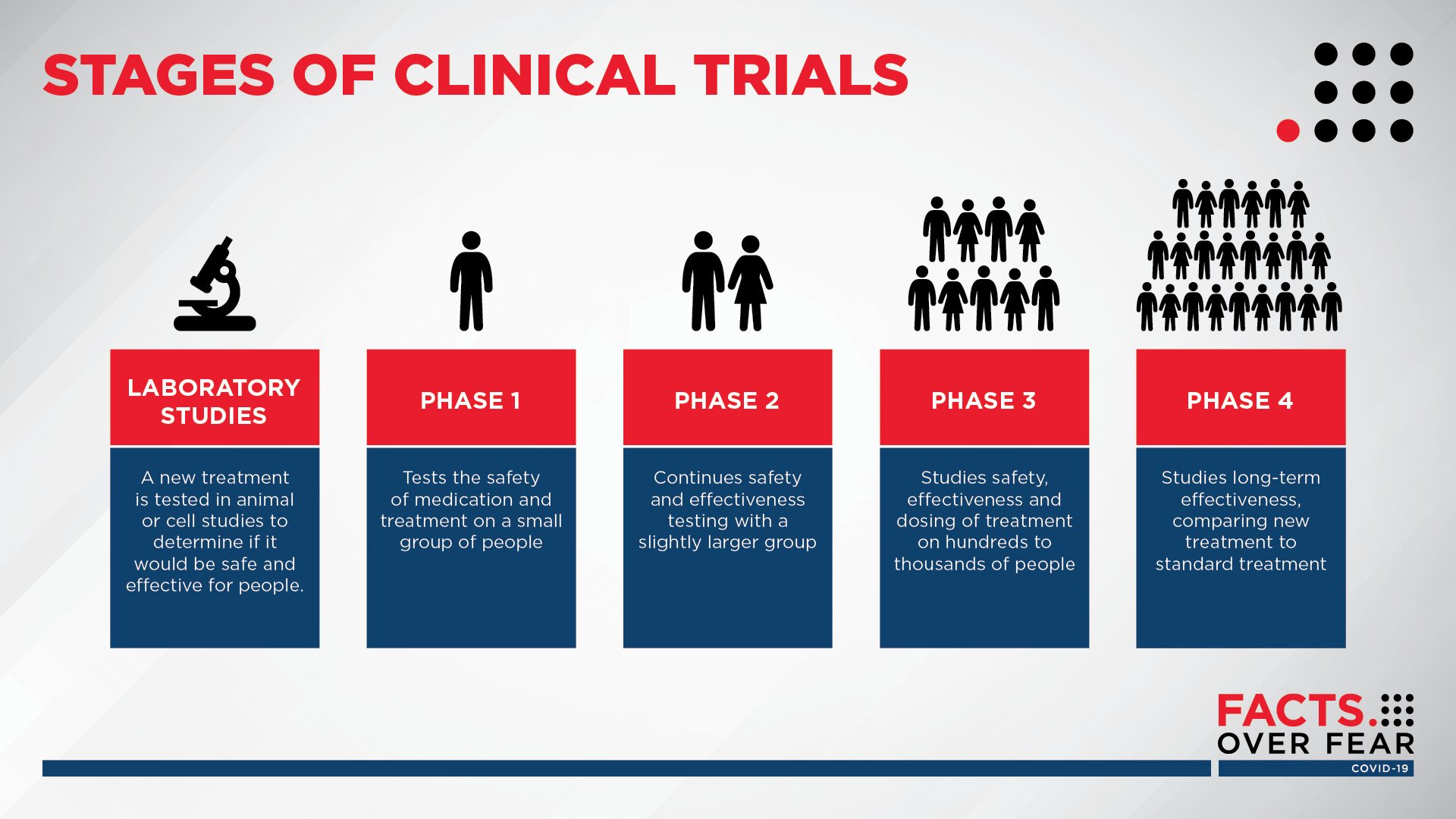
(Infographic by Sanford Health)
“Our physicians have done a phenomenal job in terms of understanding what is the best care for someone who has COVID right now.”
While some are more likely to face serious illness, he says exploring additional treatment options may be necessary, for example, those with a weakened immune system, hypertension, cardiovascular or kidney disease.
“Our initial focus has been developing clinical trials who can aid and help people that may be more susceptible to attributes of the disease.”
Learn more: Find a Sanford Research clinical trial that’s right for you
Developing a vaccine
More than 170 vaccines are being developed for testing in experiments over the world, experts say. At this point, no vaccine has reached FDA approval.
Enlarge
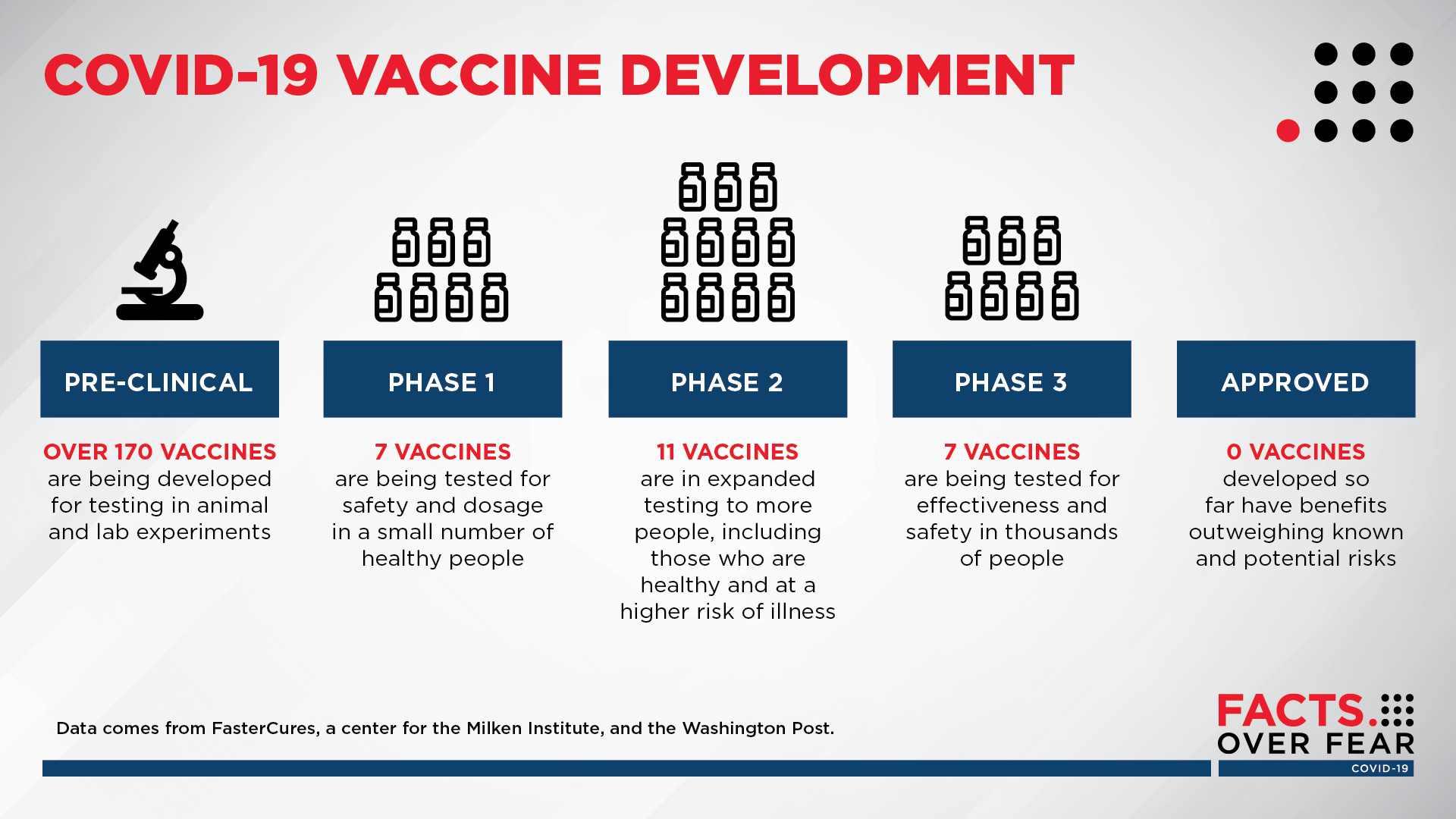
Infographic by Sanford Health
The big question is distributing that vaccine to consumers and convincing them to get one.
“Will we have enough doses for everyone on the planet or in the United States?” Dr. Pearce explains. “It’ll be interesting because so much is being invested in research and the same investment will happen in manufacturing and distribution.”
Sanford Health will be very active in that process, he says.
Follow us on Facebook as Sanford Health News continues the conversation with Sanford Health leaders.
Read more
- Clinical trial begins for critically ill COVID-19 patients
- COVID-19 Q&A: Sanford Research on vaccine, finding a cure
- Sanford Research: A leader in science, eliminating diseases
…
Posted In COVID-19, Expert Q&A, Research, World Clinic
